Pharmaceutical Chemistry 2017

Theme: Novel approaches, trends and challenges in Drug Delivery Systems
Pharmaceutics is the applied science of dosage form design. In that preformulation study is a stage of development process in which the physicochemical properties of drug substance are characterized. These Preformulation studies are an important component of drug development wherein it helps development of formulations, for various stages of clinical trials. It provides the scientific basis for formulation development. It assists scientists during screening process of lead candidates based on their physicochemical and biopharmaceutical properties. This data is useful for selection of new chemical entities (NCEs) for preclinical studies which is a major section of drug development.
Relevant Conferences:
The 1st AAPS Regional One-Day Preformulation Forum June 12, 2017 North Wales, PA; 19th International Conference on Pharmaceutical Preformulation and Clinical Pharmacy March 23 - 24, 2017, Prague, Czech Republic; 7th Drug Formulation, Solubility & Bioavailability March 26-28, 2018, Boston, MA; Preclinical Form & Formulation for Drug Discovery June 4-9, 2017 Stowe, VT; 3rd Annual Formulation & Drug Delivery Congress 8-9 May 2017 London, UK; Pharmaceutical Freeze Drying Technology MAY 8- 9, May, 2017 London, UK.
Related Societies:
Congress on Innovation in Drug Delivery-APGI, Controlled Release Society-CRS, International Society for Aerosols in Medicine -ISAM, The Pharmaceutical and Healthcare Sciences Society-PHSS.
Formulation is the process in which different chemical substances i.e. active pharmaceutical ingredient will combined with other excipients to produce a medical compound. The instability nature of the new drug changed its desired form into undesired form when it is combined with other excipients. Now-a-days numerous advancements have been achieved in this field which in turn aids to save the time and money and encounter the challenges during formulation development.
Relevant Conferences:
The 1st AAPS Regional One-Day Preformulation Forum June 12, 2017 North Wales, PA; 19th International Conference on Pharmaceutical Preformulation and Clinical Pharmacy March 23 - 24, 2017, Prague, Czech Republic; 7th Drug Formulation, Solubility & Bioavailability March 26-28, 2018, Boston, MA; Preclinical Form & Formulation for Drug Discovery June 4-9, 2017 Stowe, VT; 3rd Annual Formulation & Drug Delivery Congress 8-9 May 2017 London, UK; Pharmaceutical Freeze Drying Technology MAY 8- 9, May, 2017 London, UK.
Related Societies:
Congress on Innovation in Drug Delivery-APGI, Controlled Release Society-CRS, International Society for Aerosols in Medicine -ISAM, The Pharmaceutical and Healthcare Sciences Society-PHSS.
Drug delivery is the method or process of administering pharmaceutical compound to achieve a therapeutic effect in humans or animals. Many medications such as proteins and peptides, antibody, vaccine and gene based drugs, may not be administered by these routes because they might be susceptible to enzymatic degradation due to molecular size etc. The conventional dosage forms provide drug release immediately and cause fluctuation of drug level in blood depending upon dosage form. Therefore to maintain the drug concentration within therapeutically effective range needs novel drug delivery system. NDDS is advanced drug delivery system which improves drug potency, control drug release to give a sustained therapeutic effect, provide greater safety finally it is to target a drug specifically to a desired tissue. NDDS is a combination of advance technique and new dosage forms which are far better than conventional dosage forms. Modes of NDDS are; Targeted Drug Delivery System, Controlled Drug Delivery System, Modulated Drug Delivery System
Relevant Conferences:
International Conference and Exhibition on Pharmaceutical & Novel Drug Delivery Systems August 07-09, 2017 Philadelphia, USA; 3rd Annual Formulation & Drug Delivery Congress 8-9 May 2017 London, UK; 2nd International Conference on Parenterals, December 05-07, 2016 Texas, USA; ; 2nd International Conference on Injectables December 05-07, 2016, Texas, USA; Respiratory Drug Delivery Conference, April 25-28, 2017 France; Drug Delivery Partnership, February 7-9, 2017 Florida USA;
Related Societies:
Congress on Innovation in Drug Delivery-APGI, Controlled Release Society-CRS, International Society for Aerosols in Medicine -ISAM, The Pharmaceutical and Healthcare Sciences Society-PHSS.
Nano Canadian Society, American Nano Society, American Society for Nanomedicine, Society for Personalized Nanomedicine.
Pharmacokinetics and Pharmacodynamics in drugs
Pharmacokinetics is the study of how is the drug absorbed, distributed, metabolized and excreted in the body. Population pharmacokinetics is the study of pharmacokinetic differences of a drug in different population groups. Clinical pharmacokinetics is defined as the applications of pharmacokinetic principles in the safe and effective management of individual patient. Toxic kinetics is defined as the applications of pharmacokinetic principles to the design, conduct and interpretation of drug safety evaluation studies.
Pharmacodynamics is the study of the impact of drugs on the body; the primary focus is the mechanisms by which the drugs exert their therapeutic & adverse effects. As the dose changes the type and degree of the response changes. Bioavailability is the proportion of the administered dose that reaches the systemic circulation.
Relevant Conferences:
Annual Drug Metabolism and Applied Pharmacokinetics Conference September 18-21, 2017 Wisconsin, USA; 13th European Congress of Clinical Pharmacology and Therapeutics June 24-27, 2017 Prague, Czech Republic; 14th Symposium on Pharmacokinetics and Drug Metabolism March 29-30, 2017 Gothenburg, Sweden; International Conference on Pharmacodynamic, Pharmacokinetic and Behavioral Tolerance May 11-12, 2017 Montreal;
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
The new drug target discovery and exploitation is a key for both the pharmaceutical industry and academic research. To ensure an insight into trends in the exploitation of new drug targets analysed the drugs that were approved by the US Food and Drug Administration.The main drawbacks in systemic drug administration are Lack of drug affinity towards the pathological site and nonspecific toxicity and other adverse effects so drug targeting may resolve some these problems. Drug target can serve both as a therapeutic approach and research tool in normal physiology and under patho-physiolocal conditions. Computer Aided Drug Design is the tool used to predict whether the small molecule will bind the target site and how strongly bind to the binding site. Molecular mechanics and molecular dynamic studies also used to estimate the interaction strength between the drug molecule and the binding site.
Relevant Conferences:
15th annual Discovery on Target September 25-29, 2017 Boston, MA; 13th European Congress of Clinical Pharmacology and Therapeutics June 24-27, 2017 Prague, Czech Republic; 14th Symposium on Pharmacokinetics and Drug Metabolism March 29-30, 2017 Gothenburg, Sweden. ; 8th International Conference on Advanced Materials and Nanotechnology February 12-16, 2017 Queenstown, New Zealand; 17th International Conference on Nanotechnology July 25-28, 2017 Pittsburgh, PA; 8th International Nanomedicine Conference July 03–05 2017 Sydney, Australia.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Route of administration is the way by which the drug molecule entering into the body. Route of administrations are basically classified based the application location of the drug molecule.The differences in routes of administration of various drugs, and the regional differences in routes of use, have implications for the provision of preventive and treatment services. Needle exchange schemes and interventions targeted at drug overdose may be more suitable in areas of high injecting prevalence. Further research into regional differences in routes of drug use should be conducted with non-clinical samples.
Relevant Conferences:
13th International Conference and Exhibition on Pharmaceutical Nanotechnology Jan 24-25, 2017 Rome, Italy; Partnerships in Drug Delivery October 19-20, 2017 MA, USA; International Symposium on Drug Delivery and Pharmaceutical Sciences March 09–10, 2017 Kyoto, Japan; Non-Invasive Delivery of Macromolecules Conference 2017 February 21-24 2017 Carlsbad, USA; 2nd International Conference on Nanomedicine, Drug Delivery, and Tissue Engineering (Nddte'17) April 4 - 6, 2017 Barcelona, Spain; Respiratory Drug Delivery 2017(RDD Europe 2017) April 25-28, 2017 Nice, ; 8th International Conference on Advanced Materials and Nanotechnology February 12-16, 2017 Queenstown, New Zealand; 17th International Conference on Nanotechnology July 25-28, 2017 Pittsburgh, PA; 8th International Nanomedicine Conference July 03–05 2017 Sydney, Australia.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
A nano drug delivery system contains a core, a particle or emulsion prepared by chemical methods act as a carrier. Therapeutic molecules and ligand are added to the core for targeting specific site. Nano drugs are distributed throughout the body via nano sized capsules. The major targets in the development of nano drugs are specific drug targeting and delivery, more safety and biocompatibility, rapid development of new medicine with a wide safety margin; and improved pharmacokinetic properties, Theoretically, nano drugs can easily pass through the fine capillary blood vessels and the lymphatic endothelium, higher binding capability and accumulation at target sites, In particular, nanotechnologies have been used to develop site-specific drug targeting, for the treatment of brain diseases and cancer.
Relevant Conferences:
13th International Pharmaceutical Nanotechnology Conference July 24-25, 2017 Rome, Italy; Partnerships in Drug Delivery October 19-20, 2017 MA, USA; International Symposium on Drug Delivery and Pharmaceutical Sciences March 09–10, 2017 Kyoto, Japan; Non-Invasive Delivery of Macromolecules Conference 2017 February 21-24 2017 Carlsbad, USA; 2nd International Conference on Nanomedicine, Drug Delivery, and Tissue Engineering (Nddte'17) April 4 - 6, 2017 Barcelona, Spain; Respiratory Drug Delivery 2017(RDD Europe 2017) April 25-28, 2017 Nice, France. ; 8th International Conference on Advanced Materials and Nanotechnology February 12-16, 2017 Queenstown, New Zealand; 17th International Conference on Nanotechnology July 25-28, 2017 Pittsburgh, PA; 8th International Nanomedicine Conference July 03–05 2017 Sydney, Australia.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Nanotechnology in Drug Delivery
Nanotechnology can be defined as a technology which can deals with study and manipulation and developing and designing particles, biomolecules of the size less than 100nm and more than 1nm with the aim of modification enhancement, lowering a particular property of a molecule or particle further which can be used in developing molecule or device.
Nanotechnology is an amalgamation of engineering science and medical sciences. Drug delivery is the one of the most promising fields of nanotechnology. Nanotechnology in drug delivery epitomized by liposomes, nanogels, Dendrimers, nanocarriers, Silicon or carbon materials, Gold nanoparticles, Fullerenes, and Magnetic nanoparticles.
Relevant Conferences:
13th International Pharmaceutical Nanotechnology Conference July 24-25, 2017 Rome, Italy; Partnerships in Drug Delivery October 19-20, 2017 MA, USA; International Symposium on Drug Delivery and Pharmaceutical Sciences March 09–10, 2017 Kyoto, Japan; Non-Invasive Delivery of Macromolecules Conference 2017 February 21-24 2017 Carlsbad, USA; 2nd International Conference on Nanomedicine, Drug Delivery, and Tissue Engineering (Nddte'17) April 4 - 6, 2017 Barcelona, Spain; Respiratory Drug Delivery 2017(RDD Europe 2017) April 25-28, 2017 Nice, France. ; 8th International Conference on Advanced Materials and Nanotechnology February 12-16, 2017 Queenstown, New Zealand; 17th International Conference on Nanotechnology July 25-28, 2017 Pittsburgh, PA; 8th International Nanomedicine Conference July 03–05 2017 Sydney, Australia.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Pharmaceutical Nanotechnology has wide applications like nanoemulsions enhancing the absorption of a drug in particular site, increasing the microbial stability of product, to develop the molecules as tracer maker in identifying the toxic materials. A large number of nanosystems have been found in pharmacy field today are liposomes, metallic nanoparticles, polymeric nanoparticles, carbon nanotubes, quantum dots, nanofibres etc. It provides opportunities to improve materials, medical devices and help to develop new technologies where existing and more conventional technologies may be reaching their limits.
Relevant Conferences:
13th International Pharmaceutical Nanotechnology Conference July 24-25, 2017 Rome, Italy; Partnerships in Drug Delivery October 19-20, 2017 MA, USA; International Symposium on Drug Delivery and Pharmaceutical Sciences March 09–10, 2017 Kyoto, Japan; Non-Invasive Delivery of Macromolecules Conference 2017 February 21-24 2017 Carlsbad, USA; 2nd International Conference on Nanomedicine, Drug Delivery, and Tissue Engineering (Nddte'17) April 4 - 6, 2017 Barcelona, Spain; Respiratory Drug Delivery 2017(RDD Europe 2017) April 25-28, 2017 Nice, France. ; 8th International Conference on Advanced Materials and Nanotechnology February 12-16, 2017 Queenstown, New Zealand; 17th International Conference on Nanotechnology July 25-28, 2017 Pittsburgh, PA; 8th International Nanomedicine Conference July 03–05 2017 Sydney, Australia.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Smart drug delivery system (SDDS) is the multi targeted, pH responsive, Stimuli sensitive delivery systems in which delivering the medication to a patient in a manner that increases the concentration of the medication in some parts of the body. Smart drug delivery systems are the future and developing effective delivery systems. Nanotechnology based smart drug delivery systems are proved efficient in diagnosis and treatment of various diseases.
Targeted drug delivery system is a special form of drug delivery system where the pharmacologically active agent selectively targeted or delivered its site action. It has been developed to optimize regenerative techniques. The system is based on a method that delivers a certain amount of a therapeutic agent for a prolonged period of time to a targeted site of action within the body.
Relevant Conferences:
13th International Pharmaceutical Nanotechnology Conference July 24-25, 2017 Rome, Italy; Partnerships in Drug Delivery October 19-20, 2017 MA, USA; International Symposium on Drug Delivery and Pharmaceutical Sciences March 09–10, 2017 Kyoto, Japan; Non-Invasive Delivery of Macromolecules Conference 2017 February 21-24 2017 Carlsbad, USA; 2nd International Conference on Nanomedicine, Drug Delivery, and Tissue Engineering (Nddte'17) April 4 - 6, 2017 Barcelona, Spain; Respiratory Drug Delivery 2017(RDD Europe 2017) April 25-28, 2017 Nice, France. ; 8th International Conference on Advanced Materials and Nanotechnology February 12-16, 2017 Queenstown, New Zealand; 17th International Conference on Nanotechnology July 25-28, 2017 Pittsburgh, PA; 8th International Nanomedicine Conference July 03–05 2017 Sydney, Australia.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
A biomaterial is any substance other than drug or combination of substances, synthetic or natural in origin. It can be used for any period of time or as a part of a system which treat diagnosis or replace any part of the body. It is used to prepare biomedical device or bio implant that is used to treat, replace or augment any tissue or organ in the body. Biomaterials are also used every day in dental applications, surgery, and drug delivery. A biomaterial is also an autograft, allograft used as a transplant material. ECM and ECM-like materials, or ECM-synthetic polymer hybrids, used as biomaterials in the field of regenerative medicine,
Relevant Conferences:
European Symposium and Exhibition on Biomaterials and Related Areas May 9-10, 2017 Weimar, Germany; 28th Annual Meeting of the European Society for Biomaterials September 04-08, 2017 Athens, Greece; Society for Biomaterials 2017 Annual Meeting April 04-08, 2017 Minneapolis, USA; International Conference on Advances in Bio-Materials and Their Applications August 31-September 1 2017 London, UK; 3rd International Symposium on Biomaterials & Biosensors (BIOMATSEN 2017) April 22-26, 2017 Turkey; IASTED International Conference on Biomaterials and Tissue Engineering (BTE 2017) February 20-22, 2017 Innsbruck, Austria.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Vaccine is a material that can produce an immunologically mediated resistance to a disease. Vaccines are prepared by killed or attenuated microorganisms. Vaccine drug delivery systems are getting popularity due to benefits they offer. Needle free technology, edible vaccines used for oral delivery of vaccines. Reason behind development of vaccines as controlled drug delivery systems are ; 1) Immunization failure with conventional immunization regimen, 2) Allow for the incorporation of doses of antigens so that booster doses are no longer necessary as antigens are released slowly in a controlled manner 3) Control the spatial and temporal presentation of antigens to the immune system there by promoting their targeting straight to the immune cells.
Relevant Conferences:
28th World Congress on Vaccines October 26-28, 2017 Paris, France; World Vaccine Congress 2017 April 10-12, 2017 Washington, USA; 20th Annual Conference on Vaccine Research April 24-26, 2017 North Bethesda, MD; BIT’s 9th Annual World Congress of Vaccine (WCV-2017) March 29-31, 2017 Beijing, China; 6th Asian Vaccine Conference (ASVAC 2017) April 27-29, 2017 Singapore, Singapore; 2017 National Adult and Influenza Immunization Summit May 09-11, 2017 Atlanta, Georgia.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Theranostics is defined as the combination of diagnostics and therapeutics. In this technology radio-nuclide labeled agents are used to diagnose the disease and subsequently use identical agents to treat the disease itself. Now-a-days this technology used in the cancer treatment as cancer theranostics. Theranostics nanomedicine may be defined as nanomedicine that combines diagnostics with therapeutics. Nanotheranostics is to apply and further develop nanomedicine strategies for advanced theranostics. It can also produce stimuli-responsive release, synergetic and combinatory therapy, siRNA co-delivery, multimodality therapies, oral delivery, delivery across the blood-brain barrier.
Relevant Conferences:
13th Pharmaceutical Nanotechnology Conference July 24-25, 2017 Rome, Italy; The 10x Medical Device Conference May 01-03, 2017 San Diego, USA; 2017 Design of Medical Devices Conference April 10, 11-13, 2017 Minneapolis, MN; 3rd Annual Compliance Online Medical Device Summit 2017 June 8-9, 2017 Boston, MA; Outsourcing in Clinical Trials Medical Devices 2017 June 27-28, 2017 Santa Clara, CA; BIOMEDevice Boston May 03-04, 2017 Boston, MA; Medtec Europe 4 - 6 April 2017 Messe Stuttgart, Germany.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Peptides and Protein Prug Delivery
Proteins and peptides are the most abundant components of biological systems. Peptides and proteins are attracting increasing in attention as therapeutics. Transdermal peptide therapeutics avoids the problems with GI tract. The clinical peptide therapeutic pipeline composed of 128 peptide therapeutics. The recent advances in the peptide and protein drug delivery systems are PEGylation and Depo-foam technology. Cell-penetrating peptides (CPPs) act as cargo carriers and constitute a current hotspot in medical research. CPPs to transport hydrophilic macromolecules into cells, thus, assist to execute biological functions. CPPs do not destroy the integrity of the cell membranes, and are considered more efficient and safe and providing new avenues for research and applications in life sciences.
12th Australian Peptide Conference October 15-19, 2017 Queensland, Australia; 25th American Peptide Symposium June 17-22, 2017 British Columbia, Canada; 10th Annual Proteins & Antibodies Congress 24-25 April 2017, London, UK; 5th Annual Macrocyclic and Constrained Peptides April 25-26, 2017 USA; TIDES: Oligonucleotide and Peptide Therapeutics April 30 - May 3, 2017 San Diego, CA; 13th Pharmaceutical Nanotechnology Conference July 24-25, 2017 Rome, Italy; 4th Annual Peptides Congress 24-25 April 2017, London, UK.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Meetings International proudly announces the Global Experts Meeting on Pharmaceutics & Drug Delivery Systems (Pharmaceutics Meeting 2017) scheduled during October 12-13, 2017 at Osaka, Japan. With a theme of "Novel approaches, trends and challenges in Drug Delivery Systems".
Meetings International provides a Global Platform for Pharma, Biotech, Medical and Healthcare Professionals to Exchange Ideas, Knowledge and Networking at its 100+ International Conferences.
The popularity of Formulation and Drug Delivery has increased significantly in recent years. The Drug Delivery Technology market is expected to reach USD 1,504.7 Billion by 2020 from USD 1,048.1 Billion in 2015, growing at a CAGR of 7.5% from 2015 to 2020. Drug delivery technology market offers a promising approach for the delivery of various kinds of drugs that have different molecular formulation. Drug delivery technology is aimed at maximizing the drug delivery at the targeted site so as to increase the efficiency of drug and proposing improved patient compliance.
Pharmaceutics Meeting 2017 will bring together key decision makers and innovators within this rapidly growing field. This intensive 2-Day program will examine various formulation and drug delivery strategies. The event will cover Pre-Formulation, Formulation Aspects, Pharmacokinetics and Pharmacodynamics, Drug Targeting, Drug Delivery Routes, Nano Drug Delivery Systems, Nanotechnology in Drug Delivery, Pharmaceutical Nanotechnology, Smart Drug Delivery Systems, Applications of Biomaterials, Theranostics and Peptides and Protein Drug Delivery.
Additionally, the event will examine novel strategies for improving Vaccine Drug Delivery Systems, including stability and half-life will also be discussed along with novel routes of administration. Case studies will be presented that illustrate the progress made in developing efficacious drug therapeutics, while leaders in the field point the way to the future for these promising drugs.
Join the industry’s leading drug development executives from numerous leading organizations to discuss and hear unique take-home examples, case studies and multiple drug development and delivery strategies to assist in reducing time-to-market on future drug products.
Attend to gain an unmatched experience in the Drug Delivery field.
Pharmaceutics Meeting 2017 will offer you an unmatched attendee experience. In addition to the many scientific sessions and take-home case study examples, you will leave this event with many other novel development strategies from some of our workshops and symposiums. Engage in dynamic conversation with your industry peers at our multiple networking sessions, and takeaway novel drug development and commercialization strategies, which could speed up time to market and save your organization millions. We hope you will join us in Osaka, Japan this October to enhance your drug delivery capabilities, and increase time to market on therapeutics.
Sincerely,
Operating Committee
CEO and Founder
Meetings International PTE LTD.
Market Analysis
Title: “Global Experts Meeting on Pharmaceutics & Drug Delivery Systems”
Date & Venue: October 12-13, 2017 at Osaka, Japan
Theme: "Novel approaches, trends and challenges in Drug Delivery Systems"
Summary of Pharmaceutics Meeting 2017 Conference:-
Meetings International provides a Global Platform for Pharma, Biotech, Medical and Healthcare Professionals to Exchange Ideas, Knowledge and Networking at its 100+ International Conferences. The event brings together truly innovative thinkers who are leading the way through trialing new disruptive solutions and rethinking the conventional formulation and delivery mind-set and will help you to better understand how to develop the right formulation and delivery strategy with a strong scientific, clinical and commercial mind set and how innovative scientific techniques, emerging technologies and innovative devices can transform formulation and drug delivery.
Scope and Importance:-
Pharmaceutics is the applied science of dosage form design and is the discipline of pharmacy that deals with the process of turning a new chemical entity (NCE) or old drugs into a medication to be used safely and effectively by patients. There are many chemicals with pharmacological properties, but need special measures to help them achieve therapeutically relevant amounts at their sites of action. Pharmaceutics helps relate the formulation of drugs to their delivery and disposition in the body. Pharmaceutics deals with the formulation of a pure drug substance into a dosage form. Pharmaceutics Meeting will bring together key decision makers and innovators within this rapidly growing field. This intensive 3-Day program will examine various formulation and drug delivery strategies. The event will cover the following topics.
- Formulation Aspects
- Novel Drug Delivery System
- Pharmacokinetics and Pharmacodynamics
- Drug Targeting
- Drug Delivery Routes
- Nano Drug Delivery Systems
- Nanotechnology in Drug Delivery
- Pharmaceutical Nanotechnology
- Smart Drug Delivery Systems
- Applications of Biomaterials
- Vaccine Drug Delivery Systems
- Theranostics
- Peptides and Protein Drug Delivery
The popularity of Formulation and Drug Delivery has increased significantly in recent years. The Drug Delivery Technology market is expected to reach USD 1,504.7 Billion by 2020 from USD 1,048.1 Billion in 2015, growing at a CAGR of 7.5% from 2015 to 2020.
About Venue:
Japan is a world apart – a cultural Galápagos where a unique civilization Blossomed, and today thrives in contrasts of traditional and modern. The Japanese spirit is strong, warm and incredibly welcoming. Japan is a leading nation in scientific research, particularly technology, machinery and biomedical research.
Japan leads the world in robotics production and use. Osaka is the second largest metropolitan area in Japan and serves a major economic hub. Historically a merchant city, Osaka has also been known as the “Nation’s Kitchen”. With a population of 2.5 million, Osaka is Japan’s third largest and second most important city. It has been the economic powerhouse of the Kansai region for many centuries.
The city’s west side has the main port as well as a tourist destination with attractions such as Kyocera Dome, Universal Studios Japan, Osaka aquarium, Minami, Osaka castle, Umeda sky building and the Tempozan Harbour Village. Osaka is known for its food, both in Japan and abroad. Author Michael Booth and food critic François Simon of Le Figaro have both suggested that Osaka is the food capital of the world. Osaka’s culinary prevalence is the result of a location that has provided access to high quality ingredients, a high population of merchants, and close proximity to the ocean and waterway trade. In recent years, Osaka has started to garner more attention from foreigners with the increased popularity of cooking and dining in popular culture.
Who should attend???
With members from around the world focused on learning about Pharmaceutics and Drug Delivery Systems and the most recent techniques, developments, and the newest updates in this field; Target audience expected for this conference include Pharmaceutical Industries, Drug Delivery Systems research institutes, Pharmaceutical Manufacturing Companies, Pharmaceutical Faculty, Drug Delivery Systems Faculty, Pharmaceutical and Drug Delivery Systems Associations and Societies, Pharmaceutical Researchers, Pharmaceutical & Drug Delivery Systems students, scientists, Business Entrepreneurs, Consultancies offering Pharmaceutical & Drug Delivery Systems courses and many more.
Major Nano Drug Delivery Associations around the Globe:
- American Nano Society
- European Biotechnology Thematic Network Association
- Society for Biomaterials
- Nano Canadian Society
- American Academy of NanoMedicine
- American Association for the Advancement of Science
- Nanometer-Scale Science and Technology Division of the American Vaccum Society
- NanoScience and Technology Institute
- ASME NanoTechnology Institute
- Foresight Nanotech Institute
- International Association of NanoTechnology
- The Institute of NanoTechnology
- Microscopy Society of America
- Nano Business Alliance
- European NanoTechnology Gateway
- Scottish Center for NanoTechnology in Construction Materials
- Royal Society-NanoTechnology and NanoScience
- Czech NanoTechnology Industries Association
- Erwin Schrodinger Society for NanoSciences
- Innovationsallianz Carbon NanoTubes
- NanoTechnologies for Tommorow's Society
- American Association for the Advancement of Science
Companies involved in Nano Delivery:
USA
- Oncolytics Biotech
- Bristol-Myers Squibb
- GlaxoSmithKline
- Bend Research
- Pfizer
- BioDelivery Sciences
- GE Healthcare
- Mallinckrodt plc
- Nanosphere Inc., USA
- Pfizer Inc., USA
- Merck & Co Inc., USA
- Celgene Corporation, USA
- CombiMatrix Corporation, USA
- Abbott Laboratories
- Many Major companies in the Nano Delivery market.
Global
- PolyActivaUnilife
- Mati Therapeutics
- Formac Pharmaceuticals
- Battelle
- Toxikon
- Novartis
Societies Associated with Pharmaceutical Research:-
Worldwide Associations:-
- International Pharmaceutical Federation (FIP)
- International Pharmaceutical Students' Federation (IPSF)
- European Association of Employed Community Pharmacists in Europe (EPhEU)
- European Pharmaceutical Union (EPU)
- Association of the British Pharmaceutical Industry (ABPI)
- Pharmaceutical Management Agency (PHARMAC)
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- Pharmaceutical Group of the European Union (PGEU)
- Australian College of Pharmacy
- Pharmaceutical Society of Australia
- The Pharmacy Guild of Australia
- The Society of Hospital Pharmacists of Australia
- Canadian Pharmacists Association
- Canadian Society of Hospital Pharmacists
- Chinese Pharmaceutical Association
- Danish Association of Pharmaconomists
- Indian Pharmacist Association
- Pharmaceutical Society of Ireland
- The Pharmaceutical Association of Israel
- Kuwait Pharmaceutical Association
- Pharmaceutical Association of Mauritius
- Pharmaceutical Society of New Zealand
- Norwegian Pharmacy Association
- Pakistan Pharmacists Association
- National Pharmacy Association
- Pharmaceutical Society of Northern Ireland
- Royal Pharmaceutical Society (RPS)
- American Association of Colleges of Pharmacy (AACP)
- American Pharmacists Association (APhA)
- American Society for Pharmacy Law
- American Society of Consultant Pharmacists (ASCP)
- American Association of Pharmaceutical Scientists (AAPS)
- American Society of Consultant Pharmacists Foundation
- American Society of Health-System Pharmacists (ASHP)
- Professional Compounding Centers of America
- American College of Clinical Pharmacy (ACCP)
Statistical Representation:-
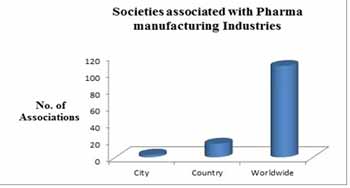
Industries Associated with Pharmaceutical Research:-
List of Pharmaceutical Companies in Osaka:
- Mikuni
- Takeda
List of Pharmaceutical Companies in Japan:
- Takeda
- Otsuka
- Meiji
- Astellas
- Daiichi Sankyo
- Teijin
- Eisai
- Chugai
- Mitsubishi Tanabe
- Kyowa Hakko Kirin
- Taisho
- Shionogi
- Hisamitsu
- Santen
- Ono
- Kyorin
- Nichi-Iko
- Mochida
- Sawai
- Kaken
- Kissei
- Zeria
- Towa
- Torii
List of Pharmaceutical Companies in the World:
- Johnson & Johnson
- Pfizer
- Novartis
- Roche
- Merck & Co.
- Sanofi
- Astrazeneca
- Abbot
- Amgen
- Sinopharm Groups
- GlaxoSmithKline
- Gilead Sciences
- Medipal Holdings
- Takeda
- AbbVie
- Teva
- Lilly
- Bristol-Myers Squibb
- Bristol-Myers Squibb
- Novo Nordisk
- Astellas
- Boehringer Ingelheim
- Actavis
- Otsuka
- Daiichi Sankyo
- Biogen Idec
- Baxter
- Merck KGaA
Statistical Representation:-
Revenue in billion U.S dollars
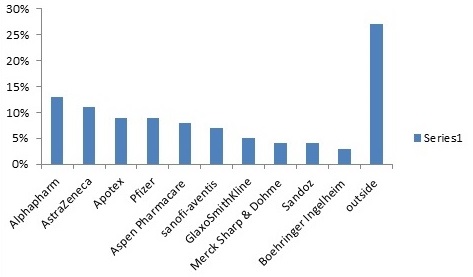
Market Value on Pharmaceutical Research:-
Worldwide Statistics:-
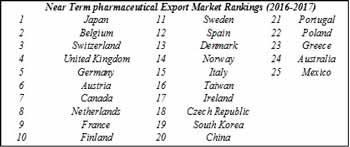
The Markets Report examines over various markets in terms of economic development, value of U.S. exports, aging populations, per capita pharmaceutical spending, degree of price controls, intellectual property protection and other factors that contribute to pharmaceutical demand growth
The global active pharmaceutical ingredients market is expected to reach USD 213.97 Billion by 2021 from USD 157.95 Billion in 2016, growing at a CAGR of 6.3% from 2016 to 2021. The factors driving market growth include increasing incidence of chronic diseases, rising prevalence of cancer, technological advancements in API manufacturing, growing importance of generics, rapidly increasing geriatric population, increase in abbreviated new drug applications (ANDA) and increasing uptake of biopharmaceuticals
- The market size of the global pharmaceutical industry is estimated to reach US$ 1.2 trillion by 2017 growing at a Compound Annual Growth Rate (CAGR) of 3-6% and the emerging markets are likely to be the key growth drivers.
- Global spending on medicines is forecast to reach nearly $1.3 trillion by 2018.
- 21 pharmerging countries will increase their contribution to growth over the next five years and account for nearly 50% of absolute growth in 2018.
- The pharmerging markets will expand at a compound annual growth rate of 8-11% through 2018.
- Total global spending will reach $1.3 trillion in 2018, an increase of $290-320 billion from 2013, driven by population growth, an aging population, and improved access in pharmerging markets.
- A compound annual growth rate of 4-7% on a constant currency basis will be slightly higher than the 5.2% recorded over the past five years.
The global nanotechnology market should reach $90.5 billion by 2021 from $39.2 billion in 2016 at a compound annual growth rate (CAGR) of 18.2%, from 2016 to 2021. The global nanocomposite market, in value terms, should reach $5.3 billion by 2021 from $1.6 billion in 2016 at a compound annual growth rate (CAGR) of 26.7%, from 2016 to 2021.
Pharmaceutical Spending – Global Market
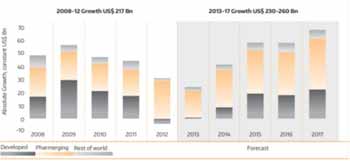
Market Growth of Pharmaceutical Research in the last and upcoming ten years
Pharmaceuticals are one of the world's most profitable industries. During the last 30 years, the industry has spent billions of dollars on research and reaped billions in return. In 2008 alone, the pharmaceutical industry sold $773 billion in products worldwide-a number that has consistently grown for the past 8 years and is projected to increase again by 2.5 to 3.5 percent in 2017, according to the drug market research firm IMS Health.
But the process that turns research dollars into medicines is a slow and often arduous one. It now takes an average of 12 to 15 years and up to $1.7 billion for a drug to go from discovery to market, according to The Pharmaceutical Research and Manufacturers of America. And despite all the time and money invested, only a handful of drugs are approved by the FDA each year.
The arduous drug approval process reveals a central fact about Big Pharma: it's one of the most intensely regulated industries in the world. The U.S. Food and Drug Administration (FDA) and its European Union counterpart, the European Medicines Agency (EMEA), govern every aspect of a drug's development-from chemicals used in the drug and clinical study instructions, called protocols, to packaging components and marketing materials. This strict oversight is meant to protect patient safety, and pharma companies take regulatory oversight seriously. The constant pressure to adhere to government mandates shapes every aspect of a pharma firm's organization, operations, and culture. For example, drug companies maintain powerful regulatory affairs divisions-the departments that deal with government agencies-and they tend to be risk-averse.
Pharmaceutical Regulations in Japan:
Manufacturing, importation, and sales of drugs and medical devices are regulated by the Pharmaceutical Affairs Law (PAL) of Japan.
All manufacturing and marketing applications in Japan for drugs and devices are reviewed by the Pharmaceutical and Medical Devices Agency (PMDA) (83). All applications are thoroughly reviewed before PMDA submits an approval recommendation to the Ministry of Health, Labour and Welfare (MHLW).
Under PAL, when importing to Japan and selling pharmaceutical products manufactured in other countries, a license for marketing authorization is required. The Marketing Authorization Holder (MAH) will be the owner of the license for marketing authorization.
The MAH must be based in Japan and can be the foreign company’s Japan office, the foreign company’s distributor, or an independent third party acting as the Designated Marketing Authorization Holder (DMAH).
To import and market a new drug in Japan, an approval (marketing approval) will be necessary. And the approval must be held by the Marketing Authorization Holder.
A foreign manufacturer intending to manufacture drugs in foreign countries and export them to Japan, is required to be accredited by MHLW as an “Accredited Foreign Manufacturer” (84). And it is necessary to obtain accreditation for each foreign factory location at which pharmaceuticals for export are manufactured.
The appointed MAH will be responsible for the labelling and advertising of the pharmaceuticals in Japan. As stipulated in PAL, the manufacturers/seller’s address, name of product, production indication, name of ingredients, expiration, etc., must be printed on the container of drugs.
Funds allotted to Pharmaceutical Research:-
Worldwide:-
Pharma companies have traditionally funded their internal R&D efforts totally on their own by investing 15% or more of their top line revenues to pay for these endeavours’. In addition, in order to tap into R&D going on outside their companies, pharma has financially supported external R&D efforts in deals with small biotech companies, research institutes, and universities. In fact, as funding for agencies like the NIH has stagnated over recent years, pharma has become an important source of funds to support early stage research.
The R&D Funding Forecast notes that there is a continuing shift in where R&D investments are being made, with fewer in the U.S. and Europe and more in Asian countries. The U.S. now accounts for less than a third of global spending, while Europe’s 34 countries account for less than 22% and Asian countries account for nearly 40%, a trend that has continued for the past five years. This trend is expected to continue through the end of the decade with China’s R&D investments surpassing those of the U.S. by about 2022.
Despite R&D budgets in the billions of dollars, pharma is now seeking to supplement their internal R&D investments with outside funding. One source has been major foundations. The recent Cystic Fibrosis Foundation investment of $58 million into Pfizer R&D to support new research into areas for this disease is a perfect example. The justification for such deals is that pharma companies have a proven R&D track record for delivering new medicines, and the foundations feel that, by tapping into these scientists, they can maximize their chances for getting breakthrough medications.
Federal Agencies:-
- Grants.gov
- NIH Grants & Funding
- USFDA
- NSF Funding
- USDA NIFA Grants
- AHRQ Funding & Grants
- PCORI Funding
- DOD Grants
- DARPA (Defense Advanced Research Projects Agency)
Non-Federal Research Associations and Foundations:
- Agency for Healthcare Research & Quality (AHRQ)
- National Pharmaceutical Council
Pharmacy research funding
Harold and Marjorie Moss Charitable Trust: -
This charitable trust awards funds for pharmacy training at undergraduate, postgraduate and doctorate level.
The Leverhulme Trade Charities Trust:-
The Leverhulme Trade Charities Trust makes grants for the benefit of chemists, commercial travellers and grocers. Pharmacists are able to apply for undergraduate and postgraduate bursaries.
The National Pharmacy Association:-
The National Pharmacy Association Health Education Foundation offers a bursary intended to support community pharmacists who wish to develop their skills to undertake research relating to community pharmacy practice.
The United Kingdom Clinical Pharmacy Association:-
The UKCPA offer a number of annual awards for various aspects of clinical practice, patient safety and IT.
Members Associated with Pharmaceutical Research:-
In regard to Industrial personnel:
Research Positions:-
- Lab technician
- Research Associate
- Research Scientist
Clinical Development and medical Jobs:-
- Clinical Research Physician
- Clinical Research Associate
- Regulatory Affairs Associate
- Bio Statistician
- Clinical Data Manager
- Medical Science Liaison
Manufacturing and Quality Assurance Jobs:-
- Process Engineer
- Quality Control Analyst
- Quality Assurance Specialist
Business Operations Jobs:-
- Market research Analyst
- Associate Product Manager
- Product Manager
- Strategy Director
- Business Development Manager
Apart from the industrial personnel where most of the research work is done, other research communities include:-
- Academicians include Student community.
- Researchers include Post docs, Research Associates.
- Scientists include Professors, Associate professors, and Assistant professor.
- Industries include Presidents, CEO’s, and R&D Managers.
Bibliography:-
http://www.ezilon.com/business/biotechnology_and_pharmaceuticals/index.shtml
http://www.wetfeet.com/articles/career-overview-pharmaceuticals
http://www.aacp.org/resources/research/Pages/ResearchFundingOpportunities.aspx
http://www.marketsandmarkets.com/pharmaceutical-market-research-3.html
http://trade.gov/topmarkets/pdf/Pharmaceuticals_Executive_Summary.pdf
- Pre-Formulation
- Formulation Aspects
- Novel Drug Delivery System
- Pharmacokinetics and Pharmacodynamics
- Drug Targeting
- Drug Delivery Routes
- Nano Drug Delivery Systems
- Nanotechnology in Drug Delivery
- Pharmaceutical Nanotechnology
- Smart Drug Delivery Systems
- Applications of Biomaterials
- Vaccine Drug Delivery Systems
- Theranostics
- Peptides and Protein Drug Delivery
- Biopolymers
- American Journal of Advanced Drug Delivery
- American Journal of Drug Delivery and Therapeutics
- International Journal of Drug Development and Research
- Research & Reviews in Pharmacy and Pharmaceutical Sciences
10 Organizing Committee Members
9 Renowned Speakers
Haya A Abubshait
Assistant Professor
Imam Abdulrahman Bin Faisal University
Saudi Arabia
Alain L. Fymat
Director (SASA)
USA
RASHID MAHMOOD
Professor
Pakistan
Seyed Abbas Shojaosadati
Professor
Iran
Nattakanwadee Khumpirapang
PhD. Student
Thailand
Roman A Melnyk
Sr. Scientist
Canada
Samira Jafari
PhD. Student
Iran
Abdul Matin
Associate Professor
Majmaah University
Saudi Arabia
Romana Zelko
Dean and Professor
Hungary